What is Water For Injection (WFI)?
Water For Injection is a solvent that is used for the manufacture of injection drugs. It can be used as an excipient as well as the final cleaning rinse agent for Bulk Active Pharmaceutical Ingredient (API) or in a Bulk Pharmaceutical Chemical (BPC) preparation.
Types of Water For Injection
- USP WFI – USP WFI is the material used to make Bacteriostatic and Sterile WFI. It is not the finished product, therefore, it needs to go through extensive validation to ensure that it meets USP parameters.
- USP Bacteriostatic WFI – Bacteriostatic WFI is sterile water that has 0.9% benzyl alcohol added as a preservative. It prolongs the water vial for up to 28 days, therefore, allowing repeated usage.
- USP Sterile WFI – Sterile Water For Injection is stored in a single-use vial and used for intravenous administration. After it is opened or heated, it cannot be reused and must be discarded.
How is Water for Injection Made?
- Distillation – According to USP, European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP) guidelines, distillation is the preferred method of manufacturing WFI for its ability to remove 100% of all impurities. Pre-treated water is boiled and the clean water vapor is collected, leaving all impurities behind. The collected vapor then undergoes condensation to return to its liquid state.
- Reverse Osmosis and Ultra-Filtration – Reverse Osmosis (RO) is permitted by USP and JP regulations, however, JP regulations require ultra-filtration if reverse osmosis is utilized. The water first goes through ultra-filters that ‘squeeze’ water and small impurities through a semi-permeable membrane. Next , the ultrafiltered water passes through a semi-permeable RO membrane which further separates the water and impurities by forcing the water through RO membranes leaving pure water.
Regulations and Sampling
As per USP < 1231>, WFI needs to meet these requirements:
| Total Organic Carbon (TOC) [µg C/L, ppb] | <500 |
|---|---|
| Conductivity at 25°C (µS/cm) | <1.3 |
| Bacteria (CFU/100 ml) | <10 |
| Endotoxin (EU/ml) | 0.25 |
These levels are monitored through test analysis and sampling periodically. Sampling is done through valves that are located throughout the system. Since WFI needs to be completely rid of microbes, the water needs to be in constant movement at 80-90°C so that microbial growth cannot occur. In order to ensure water quality is within specification, sampling is performed frequently at different use-point valves to ensure that there are no contaminants. The samples are collected and taken to a microbiology laboratory for testing per USP <1231> requirements.
Sampling Process
The collection and testing processes are critical procedures that need to be performed by highly trained technicians to avoid false-positive results due to contamination during the collection by improper techniques, poor hygienic habits, or inadequate sterilization methods. Oftentimes, companies sterilize their sampling equipment using autoclave and then create a kit for collection. Before collecting the sample, the valve is inspected for cleanliness and it is flushed for 30 seconds or more at a rate of 8 ft/s or more in order to remove bio-film structures.
The pictures below depict the sampling procedure in progress (left) and it shows an example of a use-point valve (right) from where water is collected for sampling.

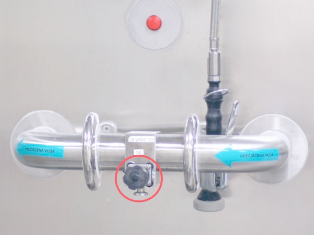
During collection, technicians have to manually place a gasket onto the flow path before clamping the sample device into the use-point valve. Placing the gasket poses a tremendous risk of contamination because the technician touches parts where the sample will flow, compromising the integrity of the results. Whenever a result is positive, costly measures are taken to investigate the source of contamination and the problem is often traced back to the collection procedure, not the system itself. This problem can be easily solved through single-use systems like our aSURE™ WFI Sampling Kit or aSURE™ Tri-Clamp Fittings. These sanitary connections come with a fused gasket, which allows the technician to successfully collect a sample without coming into contact with the fitting, removing an immense contamination risk. Our kits are gamma-radiated for microbial control, double bagged in dual laminate medical-grade pouches with easy-peel seals.
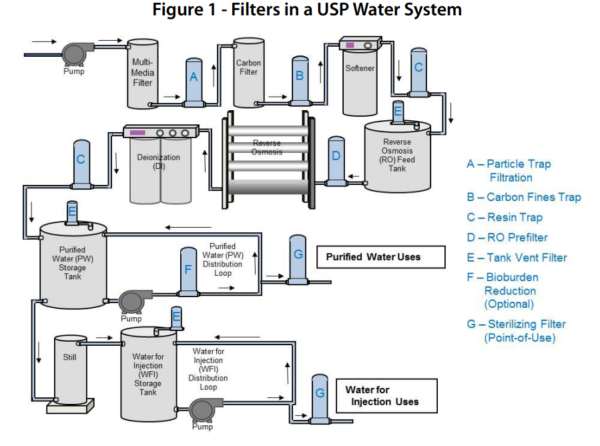
Example of a WFI Filtration and Storage System:
References
Mettler Toledo, Pharmaceutical Waters Guide, accessed 16 August 2018, <http://www.modcon.co.il/wp-content/uploads/cmdm/3793/1439448823_IB_Pharma_Waters_Guide_EN_Jan2013.pdf>.
U.S. Food & Drug Administration, 7/93, High Purity Water System, accessed on 16 August 2018, <https://www.fda.gov/iceci/inspections/inspectionguides/ucm074905.htm>.
U.S. Food & Drug Administration, 12/31/86, Water For Pharmaceutical Use, accessed on 17 August 2018,
<https://www.fda.gov/iceci/inspections/inspectionguides/inspectiontechnicalguides/ucm072925.htm>.
Pure Flow Inc., Spring 2015, How to Properly Sample Water Systems, accessed 17 August 2018, <https://www.pureflowinc.com/wp-content/uploads/2015/09/PFI-Newsletter-1503.pdf>.
United States Phamacopeia, <1231> WATER FOR PHARMACEUTICAL PURPOSES, accessed on 17 August 2018, <https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c1231_1SUSP40.pdf>.
Critical Process Filtration Inc., (n.d.), Filters in USP Water System [Diagram], accessed 17 August 2018, <https://www.criticalprocess.com/WaterTreatment/pdfs/App_Summary-Bioburden_Control_in_USP_Water_Systems.pdf>.
Additional Photo Credit: MQA Laboratories
